Bone Density Clinical Study
Evidence Sciences & RDC Clinical
LOVE YOUR BONES, STAY STRONG, SAFEGUARD YOUR FUTURE

Dr Beth Steels
When you love your bones, you safeguard your future health and wellbeing!
We all know that menopause or what some refer to as “change of life” is a completely normal part of the cycle of life. However, accepting this doesn’t make the symptoms of menopause disappear. Many women experience symptoms when the hormones estrogen and progesterone in the body drop. These reduced hormone levels can result in symptoms like hot flushes and night sweats as you’ll be aware if you’re going through menopause.
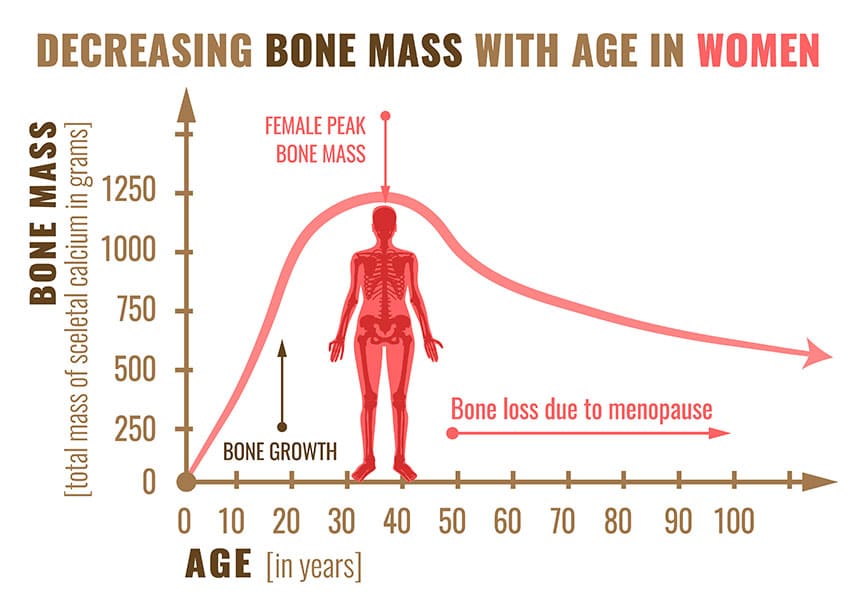
Loss of Bone Density, the silent epidemic
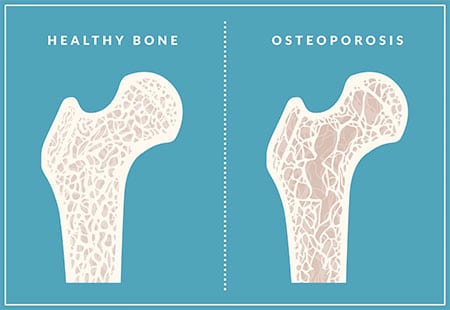
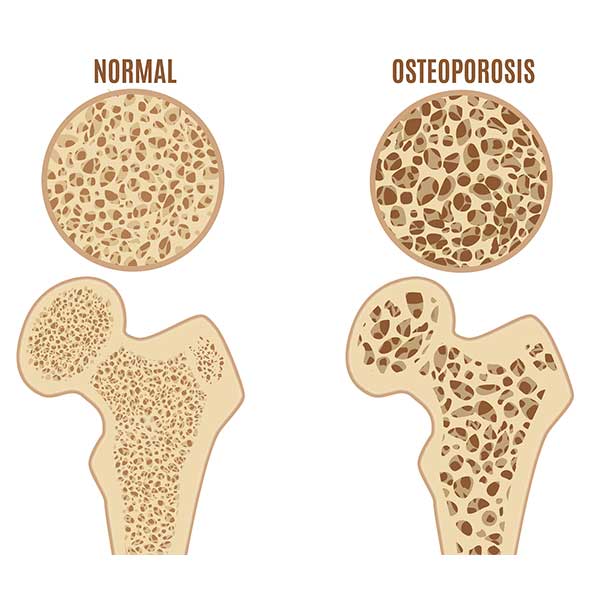
More concerning than these temporary symptoms is that menopause can trigger a rapid loss of bone density. This is especially true in the first 6 years of menopause. Loss of bone density can lead to osteopenia and osteoporosis, see below.
There are no symptoms to recognise this bone loss is happening…
Bone loss can only be detected on a bone density scan. Women at risk of losing bone density in early menopause can develop osteopenia and then osteoporosis as a result of the hormone change in early menopause. We can support bone health during menopause through health lifestyle weight-bearing exercise, vitamin D and bone supporting supplements and your GP can prescribe medications if you are at risk of osteoporosis.
The first step is having a bone density scan once menopause has started, (which is usually 12 months after the last menstrual period has occurred).

Clinical studies are a great way to get free assessment of your bone density
Our current Menopause Bone Density Study is a great way to monitor your bone density over 12 months.
If you are in the first 6 years of menopause and participate in this study the benefits to your future health and wellbeing could be immeasurable. Over the course of the study you will receive:
- Three completely free bone density scans (free) over 12 months (Start, 6 months and 12 months) to assess your bone health. Medical experts consider these DEXA scans to be the most useful, easy, and effective test for helping to diagnose osteoporosis
- Two CT scans
- You will be provided with a vitamin D supplement to also take throughout the study
- You will also receive $600 over the study to help with travel costs and your time
Recent clinical studies have shown that probiotics may maintain bone mass in menopausal women. We are testing a probiotic formulation to see if it can support your menopause symptoms and bone health over the 12 months. You may not receive the probiotic in the study, but everyone will receive it after the study.
Take part in our study and find out more about your bone density
Unfortunately if you are currently taking HRT you are not eligible for this trial
5 easy questions to see if this bone density study is for you
Support Your Community
You probably know friends and family who are going through this change of life, why not find a friend and participate together? We’re all more successful and motivated in improving our long term health and wellbeing when we team up and feel supported. Why not “buddy up” and help yourself and others enjoy life more?
What is a Dexa Scan and why is it important?
A DEXA scan is a non-invasive test that measures bone mineral density to assess if a person is at risk of osteoporosis or fracture. DEXA stands for dual energy x-ray absorptiometry—a mouthful of a term that actually tells a lot about this procedure, in which two X-ray beams are aimed at the bones. While a regular X-ray can show changes in bone density (osteopenia) after bone loss of about 40%; a DEXA scan can detect changes as small as 1%, making it more sensitive and accurate.
Your privacy
We value your privacy. You may contacted by Evidence Sciences or RDC Clinical trained trial co-ordinators will supervise your progress throughout the study. Any of your personal information collected during the study will be kept in the strictest confidence, and all data is de-identified prior to analysis. No personal details are ever published. We will not use any data supplied by you or others for any purpose other than the study in which you are involved.
Ethics approval: This Clinical study has been approved by National Institute of Integrative Medicine Human Research Ethics Committee under Approval Number: 0089E-2021.
If you need more information about our privacy policy please email our clinical trial team.

