Neuropathic Pain Study
Dr Beth Steels
NEUROPATHIC PAIN STUDY

Dr Beth Steels
Diabetic Pain Study
“Neuropathic pain is one of the most commonly experienced symptoms associated with diabetes. For the last 15 years I have been researching medicines for the management of pain and inflammation.
We now have a better understanding of the different type of pain especially diabetic related nerve pain. We are now researching and testing a novel medication specifically for this purpose.
If you suffer diabetes read on and see if you’re eligible to trial this new medication.”
Dr Beth
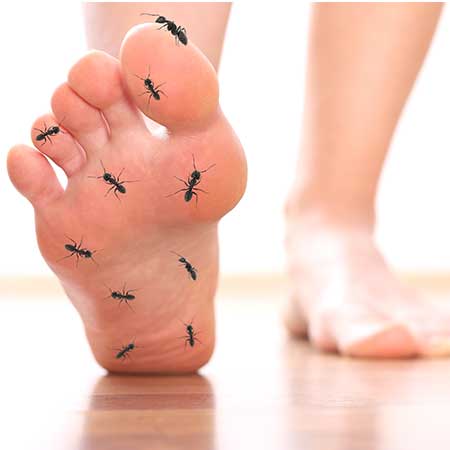
What is peripheral neuropathy?
Peripheral neuropathy commonly occurs in people with diabetes
Peripheral neuropathy refers to the pain specifically in the feet and legs, and for some people, also in the hands and fingers. The pain and the other symptoms, such as burning, pain and tingling, electric shocks, itching and sensitivity to touch, and numbness can spread to the legs or arms.
If it occurs in the legs, it can be painful at night (aching restless legs) and it can be painful to walk. It is type of pain that effects the nerves and it is different to other kinds of pain such as muscle pain.


About this trial and research group
About the research group
Research Centre: Evidence Sciences Pty. Ltd. and The University of Queensland
Location: Brisbane and Virtual via Video
Lead Researcher: Dr. Elizabeth Steels
Ethics Committee: This study has been reviewed and approved by the National Institute of Integrative Medicine Human Research Ethics Committee (EC00436)
The project is being conducted by Dr. Elizabeth Steels, Evidence Sciences Pty. Ltd. Dr Steels is the Research Director at Evidence Sciences Pty. Ltd. and an Adjunct Research Fellow, Department of Pharmacy, University of Queensland, in Brisbane. Evidence Sciences is a research and regulatory affairs consultancy firm with the primary focus on specialised nutrition and holistic wellness outcomes.
About this trial
Background
Up to 15% of people with diabetes experience varying degrees of peripheral neuropathy symptoms, including neuropathic pain, tingling, and numbness. There are very few treatments for neurological pain.
This research study is evaluating the use of a new pain-relieving nutritional supplement for neuropathic pain and inflammation in people with diabetes. The supplement is called Palmidrol, (also known as PEA or palmitoylethanolamide) and has been approved for use by the Therapeutic Goods Administration (TGA) in Australia. There are already a small number of clinical studies showing that Palmidrol is helpful for nerve (neuropathic) pain, although this will be the first study in Australia to look specifically at nerve pain in those with diabetes.
About the product
The product used in the Diabetic Pain Clinical Trial is Palmidrol (PEA), a TGA approved listed medicine. We are assessing the effect of PEA on diabetes-related nerve pain.
What will you be asked to do?
You will be asked to take a capsule, twice a day for 8 weeks and answer some questions.
How might this Diabetic Pain Clinical Trial help you?
This study may help you if your nerve pain is affecting your daily activities and sleep and is difficult to control without medications. It may also help you if you are looking for alternative medications to use to manage your pain.
Why Participate?
- You may experience potential improvements in managing your diabetes-related pain.
- If you are taking rescue pain medication, you may be able to reduce the amount needed.
- You will be helping to advance medical research.
Who Can Participate?
You can be part of the trial if;
- you are over 18 years of age
- you have diabetes and are on diabetes medication, including metformin and insulin.
- you are experiencing peripheral neuropathy
- you are prepared to be the study for 8 weeks and have a blood test at the beginning and at 8 weeks and fill out questionnaires during the study at our clinic or by secure e-medicine consult
- women must not be pregnant or breast-feeding and should not become pregnant during the research project
Your Rights
- If you decide to participate in the study and later feel that you no longer wish to be part of it, you may withdraw at any time.
- Your records relating to this study and any other information received will be kept strictly confidential, except as required by law.
- Qualified health professionals will monitor your health as it relates to the study.
What you need to know about this study
Are you eligible for this trial?
Are you are eligible for our neuropathic pain study?
Neuropathic pain is very common if you have diabetes, to find out if you’re eligible for this study see the questions below.
- Have you been diagnosed with diabetes?
- Are you taking medication to manage your diabetes (i.e. Insulin and/or Metformin)?
- Do you experience pain in your hands, fingers, legs or feet?
- Does the pain feel like and or all of these;
- Pins and needles or tingling
- Restless legs
- Burning sensations
- Numbness
- Electric shocks?
If you answered “YES” to any of these questions you could be a candidate
for our current Neuropathic Pain clinical study
Neuropathic Pain Clinical Trial
Would you like to take part in this study to see if your pain can be managed or reduced?
Click on the button below, register your interest in our trial and you’ll get a phone call from one of Evidence Sciences‘ expert practitioners to discuss your eligibility.
Enrolment for this study is now paused
Thank you for your interest we are currently evaluating responses to the study and if necessary will reopen enrolment. If you would like health advice please fill in the form here and one of our clinicians will be happy to talk to you.